http://www.csulb.edu/~cohlberg/songbook.html
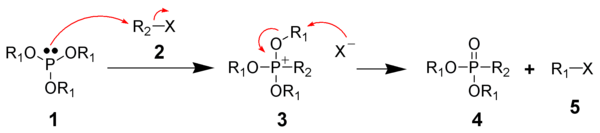
The Michaelis-Arbuzov reaction is initiated with the SN2 reaction of the nucleophilic phosphite with the electrophilic alkyl halide to give a phosphonium intermediate. Triaryl phosphites, which are unable to perform the second step of the Michaelis-Arbuzov reaction, have been shown to produce stable phosphonium salts.Likewise, aryl and vinyl halides are unreactive towards phosphites.
The displaced halide anion reacts via another SN2 reaction with the phosphonium intermediate to give the desired phosphonate and another alkyl halide.
When chiral phosphonium intermediates are produced, it has been shown the halide substitution proceeds with inversion of configuration, as expected by a SN2 reaction.
I used to understand this…. not anymore…
http://www.csulb.edu/~cohlberg/Songs/Michaelis.mp3